Nuvaxovid
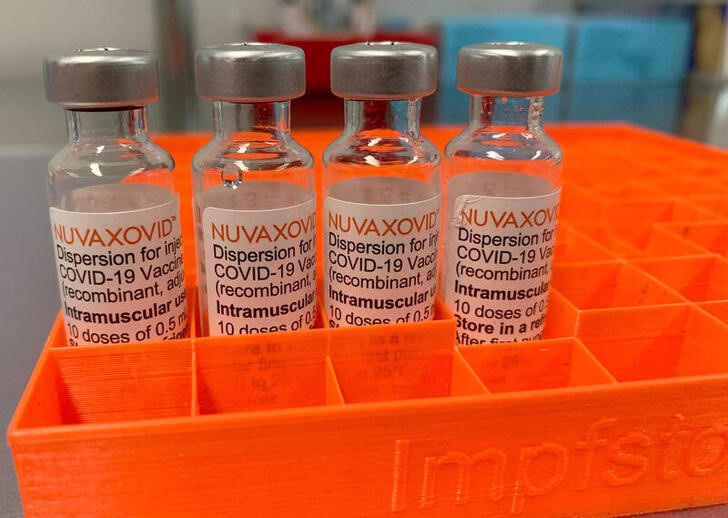
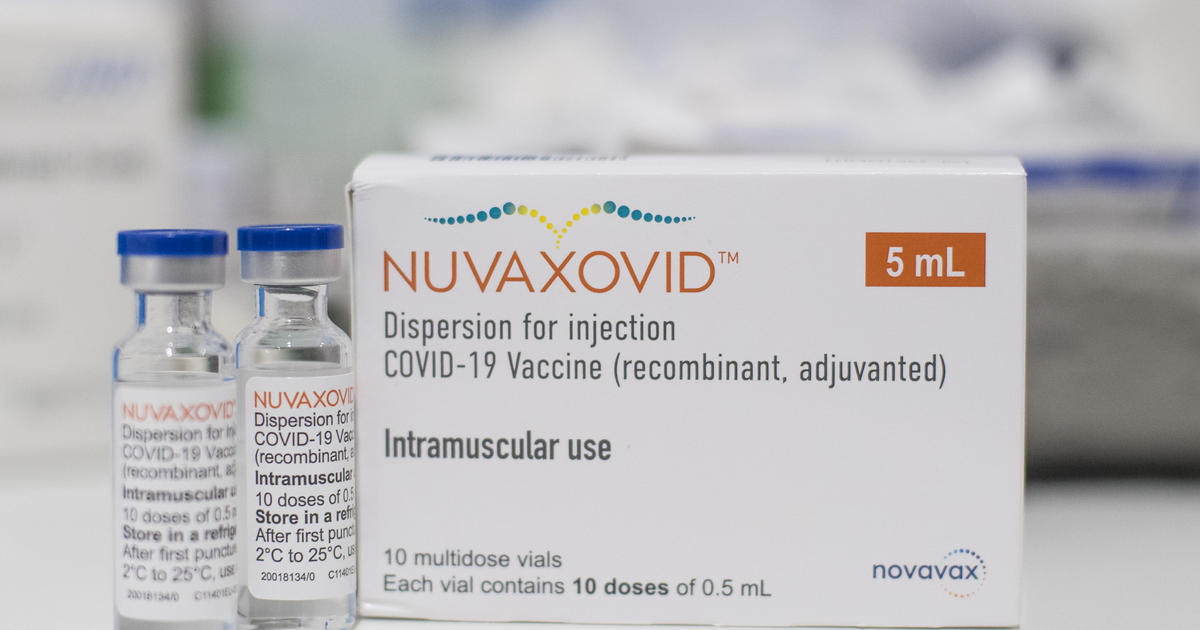
The addition of the saponin-based. This is a multidose vial.
U S Fda Authorizes Novavax Covid Vaccine For Adults Reuters
This will enable us to start offering the Nuvaxovid.
. The Novavax Nuvaxovid COVID-19 vaccine was authorized for use in Canada under the Food and Drug Regulations. 88 experienced pain. Beslutet är temporärt och gäller från.
Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30. Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.
Like the Novavax vaccine side effects were more. Nuvaxovid Novavax is approved and available for use as a primary course in people aged 12 years and over. COVID-19 Vaccine recombinant adjuvanted 2.
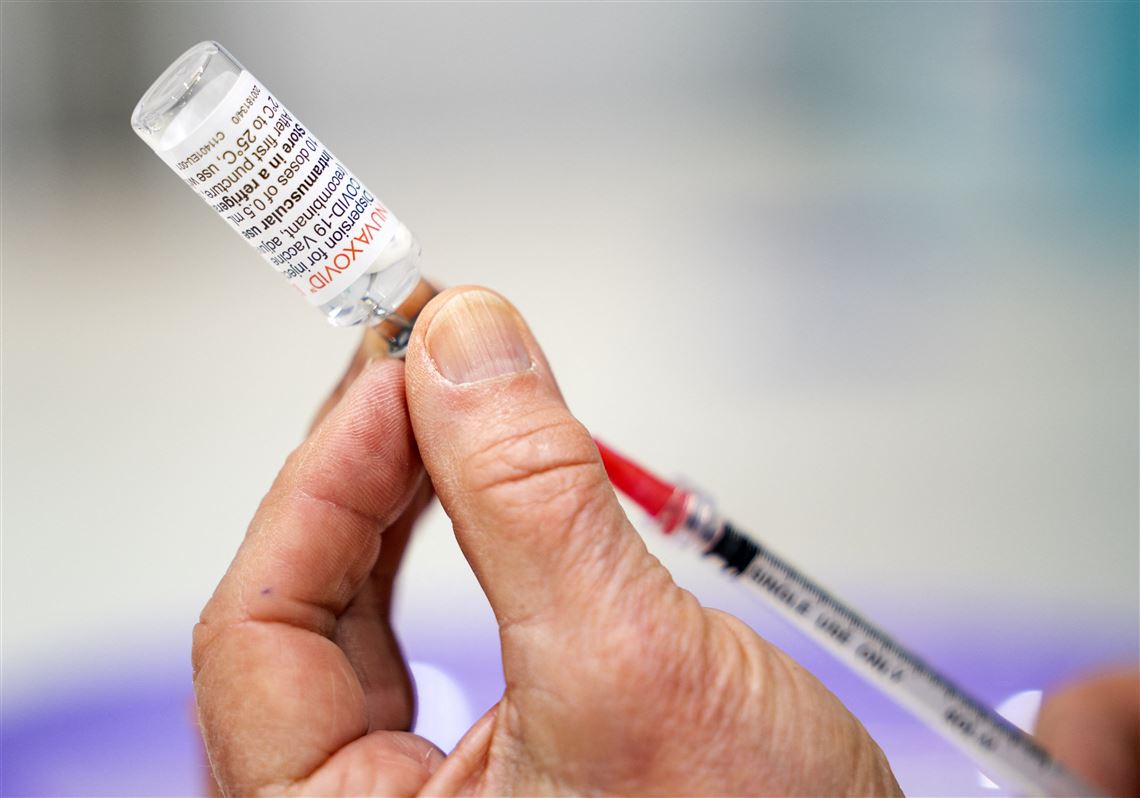
Nuvaxovid is administered intramuscularly as a course of 2 doses of 05 mL each. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. Nuvaxovid is given as two injections usually into the muscle of the upper arm 3 weeks apart.
2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. The Summary of Product Characteristics is a description of a medicinal. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19.
A booster dose of Nuvaxovid may be given to people aged 18 years and. Sverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. Rokotteesta ei myöskään ole haittaa vaikka.
Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022. Není známo zda se vakcína Nuvaxovid vylučuje. On December 20 2021 the.
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. Det proteinbaserade covid-19-vaccinet Nuvaxovid ska inte ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022. Novavax is approved and available for use as a booster in. Company Novavax should not be given to individuals younger than 30 the Public Health.
Clinical trials showed that the vaccine has around 90 efficacy in. Det eftersom att data från Australien gett signaler. The first batch of Nuvaxovid is expected to arrive in.
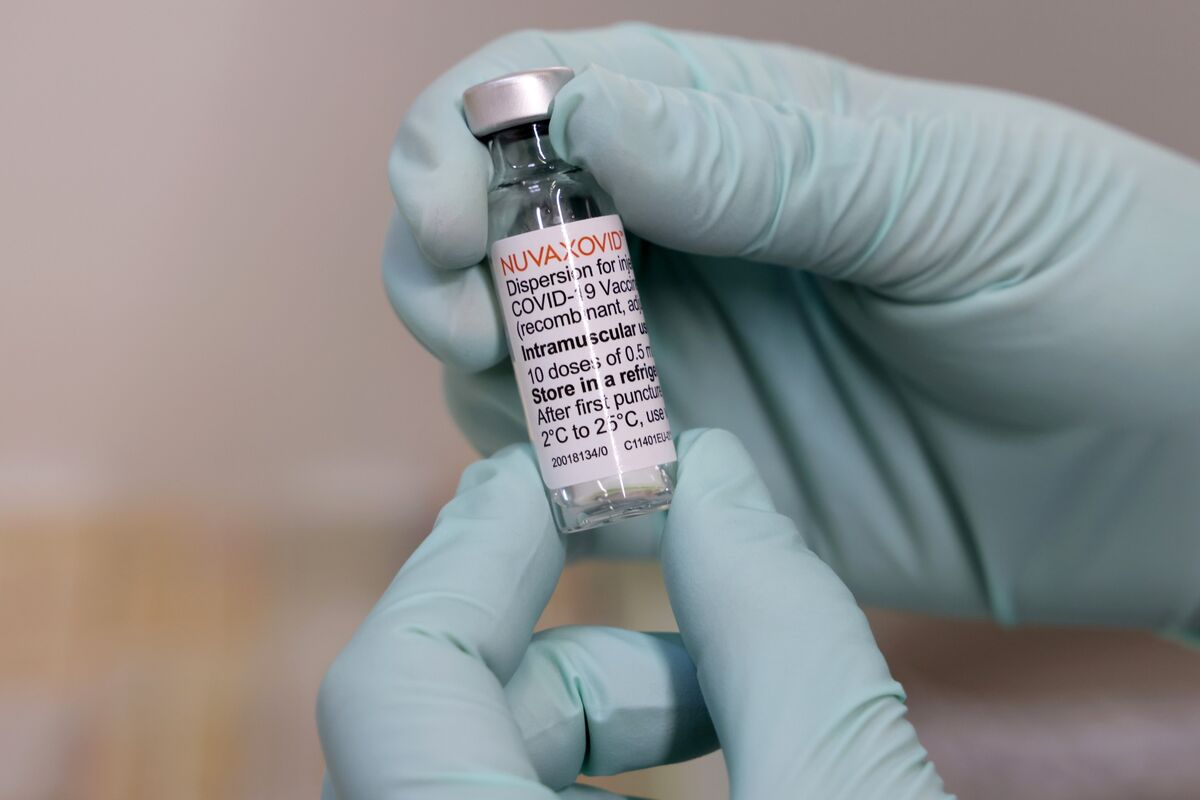
Nuvaxovid dispersion for injection. Name of the medicinal product. Nuvaxovid contains a version of a protein found on the.
16 fever including 14 severe cases. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency EMA earlier. It is recommended to administer the second dose 3 weeks after the first dose see section 51.
Nuvaxovid-rokote sopii lähes kaikille aikuisille. Qualitative and quantitative composition. Podávání vakcíny Nuvaxovid v těhotenství má být zváženo pouze v případě že možné přínosy převáží možná rizika pro matku a plod.
Find detailed technical information such as the product monograph and. Nuvaxovid is the first protein-based COVID-19 vaccine granted.
Japan Approves Novavax As 4th Covid Vaccine Amid New Surge Bloomberg
Fda To Authorize Novavax S Covid 19 Vaccine Politico

Novavax Requests Expanded Emergency Use Listing With Who For Nuvaxovid Covid 19 Vaccine For Adolescents Aged 12 Through 17 Eatg

My Kingdom For A Booster Shot Hiccups Are Yet Another Vaccine Trial Hassle Medpage Today
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News

Could The Novavax Vaccine Help Us Win This Covid War Medpage Today
Dossier Coronavirus Sars Cov 2 And Covid 19 Suspected Adverse Reactions Reported In The First Three Months Since Start Of Vaccination With Nuvaxovid Paul Ehrlich Institut

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre

Nuvaxovid Novavax S Covid 19 Vaccine Approved For 18y And Older The Immunisation Advisory Centre
Novavax Covid 19 Vaccine Nuvaxovid Approved By Mhra Pharmatutor

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care

Novavax Nuvaxovid Covid 19 Vaccine Granted Expanded Conditional Marketing Authorization In The European Union For Use As A Booster For Adults Aged 18 And Older Sep 12 2022
Nuvaxovid Por Novavax Agencia Espanola De Medicamentos Y Productos Sanitarios
Covid Vaccine Maker Novavax Drops After Cutting Sales Outlook 50 Nvax Bloomberg

Novavax Eyeing The Covid Vaccine Hesitant And Kids Unveils New Education Campaigns As Nuvaxovid Nears Us Finish Line Fierce Pharma

Ema Recommends Nuvaxovid For Authorisation In The Eu Certifico Srl

Novavax S Vaccine Nuvaxovid Vaktsineeri Ee
Novavax Sees Conditional Approval Of Covid 19 Vaccine Booster In Australia As Us Fda Delays Its Decision
